Corrosion behavior arising from biofeedstocks
Biofeedstocks represent an important sustainable fuel source, with potential to reduced green house emissions and dependency on oil. However, these feedstocks (e.g. soybean oil, palm oil, used cooking oils, etc) present unknown corrosion risks to processing and transport infrastructure. This collaborative effort aims to correlate molecular structure, chemistry and reaction pathways (in solution and at the metal-organic interface) to anticipate corrosion behavior.
A more complete description to come.
See our recent publication in Corrosion Science: https://doi.org/10.1016/j.corsci.2023.111088
Oxidation of transition metal diboride thin films
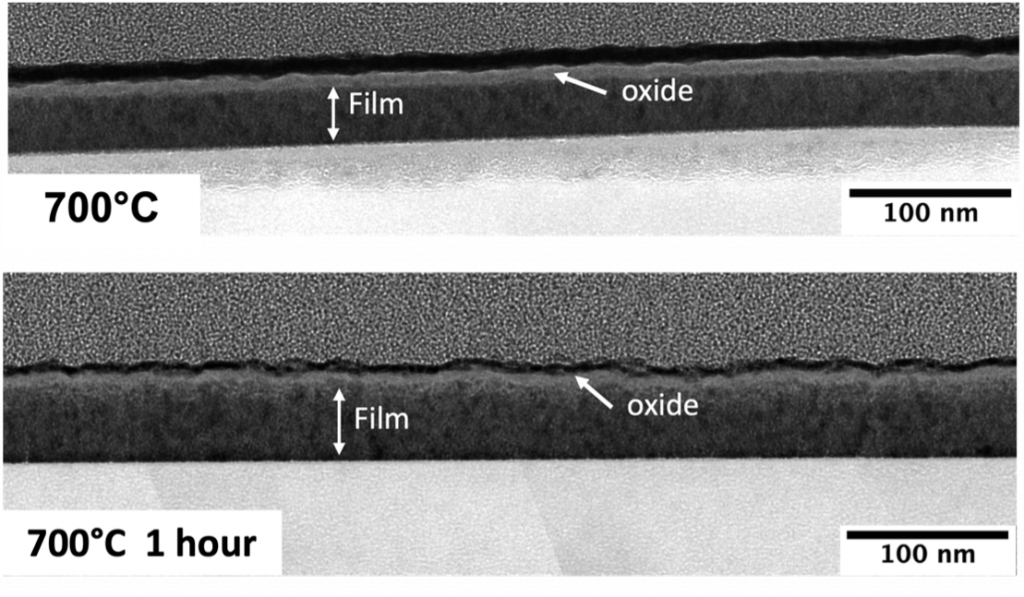
Diborides are ultra-high temperature ceramics that are known for their hardness, wear resistance and stability in extreme chemical and thermal environments. For bulk diborides, the oxidation resistance at high temperatures stems from the formation of a surface layer of liquid boron oxide. This boron oxide is volatile above 700°C, especially in the presence of moisture, and the evaporation of boron oxide at higher temperatures leads to the gradual consumption of the bulk ceramic. However, this poses a problem for the volumetrically limited transition metal diboride coatings and thin films. These lucrative properties of diborides make them extremely suitable coating materials for high temperature applications, but oxidation limits their application temperature regime, especially for application temperatures around 700°C.
Check the Complete Research Projects for more corrosion and oxidation projects.