Reduced Emissions, Alternative Energy Generation & Energy Storage
Developing low cost-austenitic steels for hydrogen facing technologies
Austenitic stainless steels are already widely used for hydrogen applications on the basis of their high resistance to embrittlement at high operating hydrogen pressures. But relative to ferritic systems, they suffer from low strength and high cost, up to a 10-fold increase driven by the Ni content. Moreover, austenitic steels are still susceptible to embrittlement, with ductility loss found to be most severe at about 200K.[1] This phenomenon is not well explained by classical extrinsic descriptors meaning that, currently, there is no physics-based path for development of improved alloys.
A phenomenon known as Hydrogen Enhanced Localized Plasticity (HELP)[2],[3] has been shown to facilitate the development of the dislocation microstructure at strains much smaller than those associated with similar microstructures in the absence of hydrogen.[4],[5] All hydrogen-induced failures of austenitic systems, ductile, intergranular, or twin boundary are associated with HELP. These failures cannot be avoided when hydrogen facilitates shear localization, even in stable austenitic microstructures. In fact, blocking localized slip with ε-martensite was found to lead to a more fracture resistant alloy than one with fully stabilized austenite.[6]Given that control of HELP and localized slip is the means to manage and even remediate hydrogen embrittlement, we will focus attention on understanding the transitioning of the dislocation microstructure from tangles to slip banding and cell formation. Such an approach will enable the development of a constitutive model whose foundation will be the dislocation microstructure and its evolution.
It has been posited that hydrogen embrittlement of austenitic stainless steels is advanced by: i) hydrogen-induced reduction of the stacking fault energy (SFE), related to composition and temperature, which hinders dislocation cross slip, thus enhancing slip planarity, ii) the presence of α’ martensite, related to the stability of the austenite, whose tetragonal body-centered structure may promote embrittlement by offering rapid diffusion paths for hydrogen, and iii) hydrogen-induced shear localization as opposed to homogeneous deformation. However, there is no conclusive evidence that SFE reduction controls slip planarity given its small magnitude (~35 mJ/m2) in this class of steels, hydrogen fractures in stable and metastable alloys have been found to be similar, namely martensitic transformation or twining are not a required conditions for embrittlement, and slip localization can take place in these steels even in the absence of hydrogen, although at less intensity.[7] Meanwhile, the role of SRO, which is defined as any local deviation from a random solid solution, its dependence on alloy composition, and its correlation to plastic shear localization in austenitic steels has received limited attention. Planar glide is frequently reported in alloys known to exhibit SRO, especially those containing higher levels of nitrogen,[8] but the extent of SRO or the degree of dislocation-SRO interactions has not been quantified. As it is currently presented in the literature the role of SRO in hydrogen embrittlement lacks nuance—it is broadly presupposed that the presence of any SRO former, must cause localized slip.[9] Our recent efforts have revealed SRO behavior in several common commercial austenitic steels via electron microscopy, that such SRO domains are expected to dramatically increase the stress necessary for dislocation motion via continuum modeling and that H has a propensity to segregate to such SRO domains via atomistic modeling. All of these observations reinforce our original postulate that SRO may be a key factor in the hydrogen embrittlement of austenitic steels. We anticipate that with improved understanding of the underlying mechanisms for SRO formation and the transition from homogenous to localized slip in the presence of H, it is possible that the strength of SRO can be tuned to anticipate the effects of H thereby preventing the transition to localized deformation. Importantly, this understanding would expand the available alloying choices and allow for cost savings by reducing concentrations of valuable metals, such as Ni or Mo, and through improved properties that enable less conservative designs.
This is a highly collaborative project involving contributions from:
Jessica A. Krogstad, Po-Cheng Kung, Quinten Yurek, Tianyu Su
Department of Materials Science and Engineering, University of Illinois Urbana Champaign
Kshitij Vijayvargia, Zahra Hosseinisarani, Elif Ertekin, Brian Somerday, Petros Sofronis
Department of Mechanical Science and Engineering, University of Illinois Urbana Champaign
Hoon Lee, Dominic Piedmont, James Stubbins
Department of Nuclear, Plasma and Radiological Engineering, University of Illinois Urbana Champaign
Mohsen Dadfarnia
Department of Mechanical Engineering, Seattle University
Toshihiro Tsuchiyama
Department of Materials Science and Engineering, Kyushu University
As well as our partners at Sandia National Laboratory, Livermore (C. San Marchi), Arcelor-Mital, Linde and Swagelok.
Find our most recent Annual Merit Review Presentation here.
[1] Jackson, H., San Marchi, C., Balch, D. K., Somerday, B. P., and Michael, J. (2016) Metall. Mater. Trans. A, 47(8), pp. 4334–4350
[2] Dadfarnia, M., Nagao, A., Wang, S., Martin, M. L., Somerday, B. P. Sofronis, P. (2015), Int. J. Fract. 196, 223-243.
[3] Martin, L. M., Dadfarnia, M., Nagao, A., Wang, S., Sofronis, P. (2019) Acta Mater.
[4] Martin, L. M., Robertson, I. M., Sofronis, P. (2011) Acta Mater, 59, 3680-3687
[5] Martin, L. M., Somerday, B. P., Ritchie, R. O., Sofronis, P., Robertson, I. M. (2012), Acta Mater, 60. 2739-2745.
[6] Koyama, M., Okazaki, S., Sawaguchi, T., and Tsuzaki, K. (2016) Metall. Mater. Trans. A, 47(6), pp. 2656–2673.
[7] San Marchi, C. (2012) in Gaseous Hydrogen Embrittlement of Materials in Energy Technologies, (Woodhead Publishing Limited).
[8] Vogt, J-B. Journal of materials processing technology 117.3 (2001): 364-369.
[9] Michler, Thorsten, and Jörg Naumann. International Journal of Hydrogen Energy 35.3 (2010): 1485-1492.
Understanding Short-range Order in Austenitic Steels for use in Hydrogen-Facing Applications
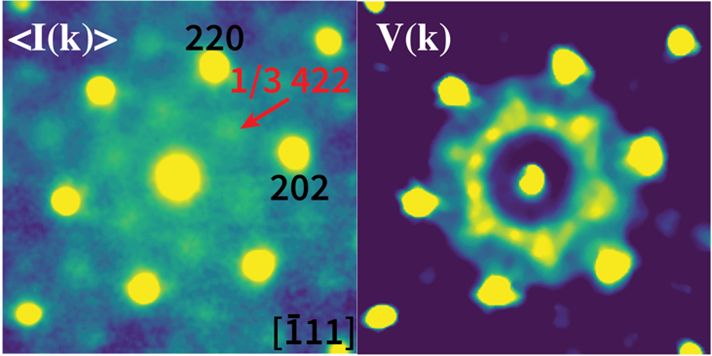
This project was motivated by the demands for improved hydrogen resilience in austenitic steels as described above. For more details about how this technique intersects with the goals of the project, please check out the linked AMR presentation for additional details.
Electron diffuse scattering caused by short-range ordering (SRO) in austenitic steels has been observed using traditional selected area electron diffraction. However, such technique struggles to differentiate diffuse scattering signals stem from different types of SROs (heterogeneous and homogeneous SRO) in the system and thus limits the complete understanding of SRO behaviors. In this work, we demonstrate a procedure utilizing Scanning Electron Nanobeam Diffraction (or 4D-STEM) method and a basic Fluctuation Electron Microscopy technique, variance map, to analyze different type of SROs in a solution treated austenitic steel. The combination of 4D-STEM and variance map techniques enables SRO domain identification and deconvolution of homogeneous and heterogeneous SRO diffuse scattering.
More recent details about this method were presented at Microscopy & Microanalysis 2023 in Minneapolis, MN. Click here for the full abstract. A full manuscript will be submitted in Fall 2023.
Understanding transport mechanisms and mechanical properties in novel ion conducting ceramics
Developing novel solid electrolytes and exploring mechanical incompatibilities and failure modes at interfaces within batteries and other energy storage technologies.
To learn more about our efforts in developing and characterizing novel solid electrolytes check out these recent publications by Dr. Yu-Ying (Steven) Lin:
- Yu-Ying Lin, Carlos Juarez-Yescas, Kai-Wei Lan, Paul V. Braun, Jessica A. Krogstad, Nicola H. Perry. “Isolation of Grain vs. Intergranular Transport in Li1+xTixTa1-xSiO5 Reveals Concerted Ion Migration in a High-voltage Stable Electrolyte from High-Throughput Descriptors.” Under Review at ACS Applied Energy Materials Summer 2023.
- Y-Y. Lin, J.Qu, W.J. Gustafson, P-C Kung, N. Shah, S. Shrivastav, E. Ertekin, J.A. Krogstad, N.H. Perry. “Coordination flexibility as a high-throughput descriptor for identifying solid electrolytes with Li+ sublattice disorder: A computational and experimental study.” J Power Sources 553 (2023) 232251. DOI: 10.1016/j.jpowsour.2022.232251
- Y-Y. Lin, A.X.B. Yong, W.J. Gustafson, C.N. Reedy, E. Ertekin, J.A. Krogstad, N.H. Perry. “Toward design of cation transport in solid-state battery electrolytes: Structure-dynamics relationships.” Current Opinion in Solid State & Materials Science 24 [6] 100875 (2020). DOI: 10.1016/j.cossms.2020.100875 (Invited Review)
This project also entails some forthcoming work exploring the degredation of porous metal anode structures during cycling. Check back soon for more details.
Corrosion behavior arising from biofeedstocks
Biofeedstocks represent an important sustainable fuel source, with potential to reduced green house emissions and dependency on oil. However, these feedstocks (e.g. soybean oil, palm oil, used cooking oils, etc) present unknown corrosion risks to processing and transport infrastructure. This collaborative effort aims to correlate molecular structure, chemistry and reaction pathways (in solution and at the metal-organic interface) to anticipate corrosion behavior.
There has been extensive research towards the development of alternative fuel sources to petroleum. Concerns regarding energy security, long-term renewability and the carbon cost of petroleum feedstocks have spurred interest in substitute feedstocks. First generation bio-feedstocks, such as triacylglycerol (TAG)-based biological oils and fats, are an essential supplement to traditional feedstocks. They are commercially attractive due to a mature supply chain and renewability. TAGs in particular have the additional advantage of being “dro- p-in” feedstocks, i.e. they can be co-refined with petroleum feedstocks without additional front-end process units or equipment upgrades. Despite the commercial success of TAGs in the energy sector, their use is currently limited by concerns regarding corrosion [2]. TAG feedstocks have substantially different chemistry than traditional petroleum feedstocks—namely, they contain a large number of oxygen-containing moieties including esters, carboxylic acids, and alcohols. These chemical differences result in a feedstock with higher reactivity, lower thermal stability, and increased hydrophilicity relative to a petroleum feedstock. These features result in increased corrosiveness of the feedstocks to- wards process equipment. Increased equipment corrosion is one of the many challenges that must be addressed as petroleum feedstocks are replaced with biofeedstocks.
Corrosion of carbon steel by triacylglycerol-based feeds is under- stood to be a complex process, wherein bulk solution reactions affect surface reactions and vice versa. And while a traditional understanding of corrosion attributes the corrosivity of a feed to a single feature (the acid content), the present results show a complex reaction space that may reveal opportunities for corrosion mitigation through continued understanding of the earliest stages of the corrosion process. Evolution of both the solution and steel surfaces was found to proceed through three distinct phases. The onset of corrosion was characterized by carbon accumulation and TAG hydrolysis. Intermediate timesteps revealed unanticipated water generating reactions and rapid iron dissolution. Finally, the longest exposures investigated did not show evidence of meaningful surface passivation—instead significant oxide growth was associated with rapidly increasing iron concentrations in solution. This suggests that despite near complete consumption of glycerol-attached ester groups, corrosion of the steel coupon progressed readily. Any efforts to anticipate corrosion behavior in more complex industrial settings or to develop mitigation strategies will depend on continued dissection of the broad progression of reactions observed and postulated by this study. In particular, the present study highlights the importance of understanding nontrivial water generation due to the presence of biofeedstocks and of alloy selection and surface chemistry in propagating corrosion reactions, while also emphasizing the need for longer-term, elevated temperature corrosion experiments to explore the possibility of eventual passivation.
For more details see Liu et al. Corrosion Science 216 (2023) 111088. DOI: 10.1016/j.corsci.2023.111088
This work is supported by the bp ICAM and continues to explore more fundamental surface reactions between organic acids and relevant structural surface chemistries.
Green Steel
The steel industry currently accounts for about 8% of global CO2 emissions, emitting 1.8 tons of CO2 per ton of steel produced. About one third of that is for making iron. This project seeks to demonstrate a process for reducing iron ore to iron that reduces cost, eliminates CO2 emissions, and increases efficiency.
Currently, more than 70% of the world’s steel is produced by the Blast Furnace (BF) – Basic Oxygen Furnace (BOF) process. In this process, iron ore is reduced using coke and limestone at temperatures above 2,000°C in the BF to create molten pig iron. Pig iron has a high carbon content which makes it brittle. To reduce the carbon content, pig iron is processed in the BOF by treating it with pure oxygen using a water-cooled lance. This removes the carbon as CO2, yielding a crude steel product. Additional processing fine tunes the metallurgy to make the final high-grade steel product. The BF process accounts for about 70% of the CO2emissions.
An alternative route for making steel is the Direct Reduced Iron (DRI) – Electric Arc Furnace (EAF) route. In the DRI/EAF process, the DRI process uses coal or natural gas to produce a mixture of H2 and CO to reduce iron ore to metallic iron. The higher reactivity of H2 allows the reduction to occur at a temperature below the melting temperature of iron (1535°C), thus a molten liquid phase does not develop. The process uses a shaft furnace with the iron ore fed into the furnace in pellet form. Natural gas DRI has the potential to significantly reduce CO2 emissions. DRI also offers the advantage of lower capital cost and complexity in design and operation compared to blast furnaces. However, shaft furnaces cannot match the production rate of blast furnaces due to problems with sticking and fusion of particles and pellet disintegration.
This collaborative effort aims to leverage H-plasmas to more efficiently reduce iron ores by reducing both the temperature and power needed direct reduction of iron (DRI) even further.
In the Krogstad group we specialize in the microstructural and chemical characterization of partially reduced ores to inform process optimization by our partners at Argonne National Laboratory and in the Department of Nuclear Plasma and Radiological Engineering at UIUC.