Ion induced amorphization and recrystallization
In situ observation of nanoparticle sintering via room temperature ion irradiation
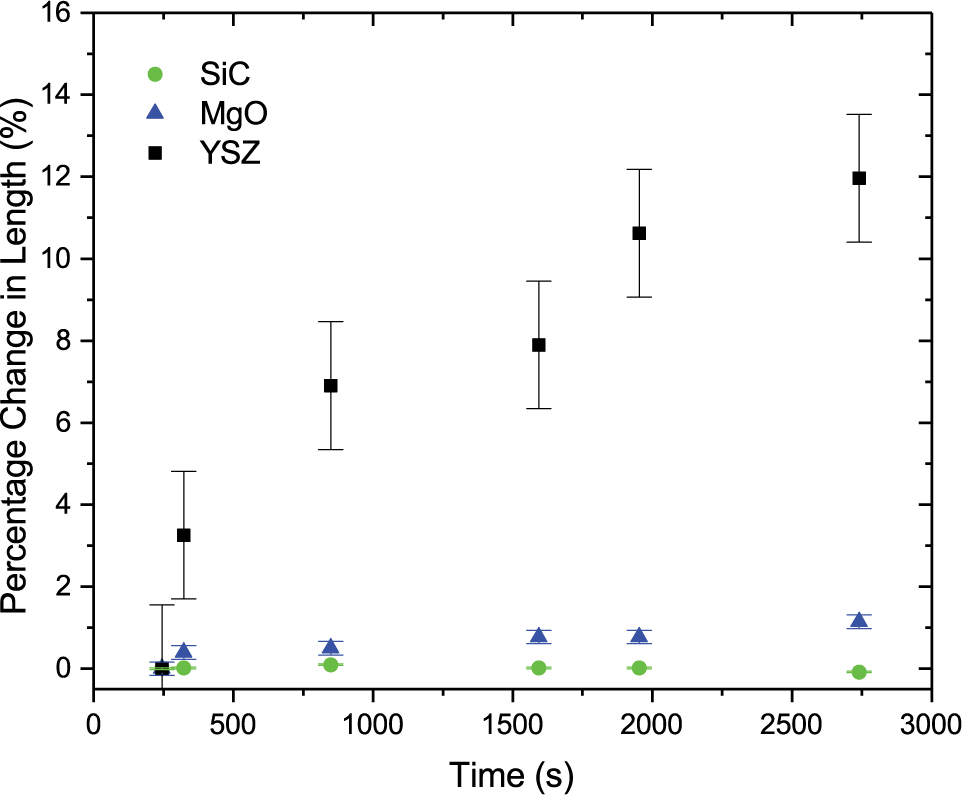
Ceramic particles typically require significant temperatures to densify or sinter–the process of joining to individual particles into one larger body. High temperatures are necessary to activate diffusion mechanisms, that allow for mass to redistribute based on driving forces such as curvature and surface energy. However, when a ceramic is irradiated with ions, collisions between the energetic ions and the atoms on the crystalline lattice result in a high density of point defects–vacancies and interstitials. Vacancy mediated diffusion is characterized by that thermal activation barrier described earlier. Interstitial diffusion, has an even higher activation barrier, but if the collision events overcome this, the actual motion of interstitial is expected to be quite easy therefore allowing mass transport at much lower temperatures. Using in situ transmission electron microscopy to observe the evolution of ceramic nanoparticles subject to ion irradiation, we can see the consequences of this interstitial diffusion–room temperature sintering! The video below, of ceria nanoparticles, is captured at room temperature and compressed to show 60 minutes of imaging in just a few seconds. Can you see how the agglomerate changes shape? Ex situ irradiation gives rise to the same evolution, so the electron beam used in imaging does not significantly contribute to the microstructural changes. Likewise, the temperature only increases by a few degrees Celsius during the experiment.
Relevant publications:
- N.J. Madden, S.A. Briggs, D. Perales, T.J. Boyle, K.Hattar, J.A. Krogstad. “Measuring radiation enhanced diffusion through in situ ion radiation induced sintering of oxide nanoparticles.” Under revision Acta Mater. 2022. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3951050
Nanoporous ceramics as a route to improved radiation tolerance
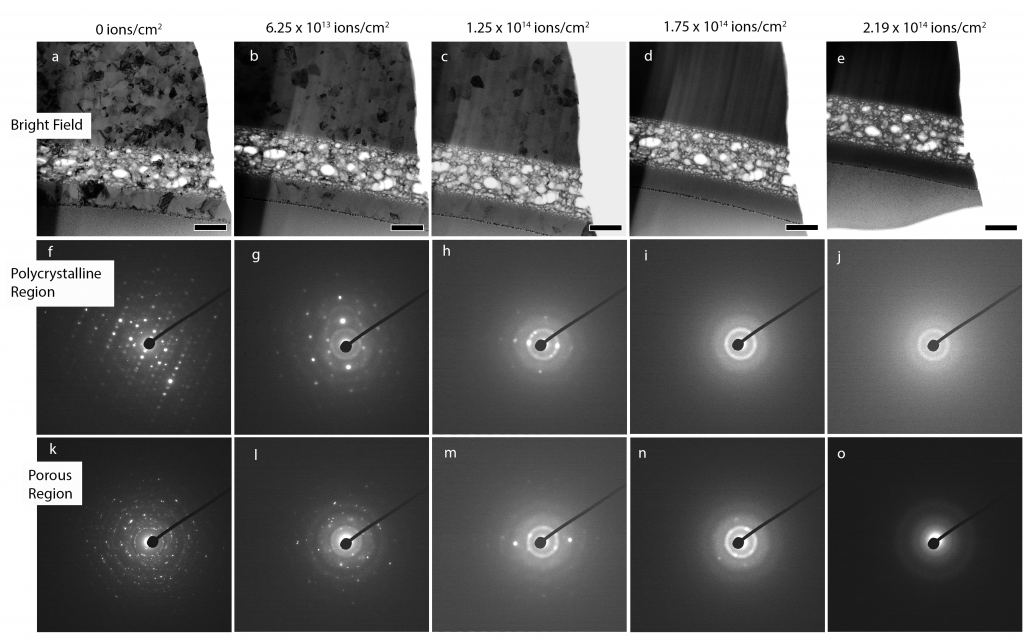
Defect sinks are key to reducing radiation induced damage accumulation; however, not all defect sinks are equally effective. The efficacy of grain boundaries and pores (i.e. free surfaces) in gadolinium titanate (Gd2Ti2O7) are directly compared via in situ ion irradiation transmission electron microscopy (TEM). A unique sample configuration is developed, in which each of three distinct regions, containing only one primary sink of interest, are contained in the same TEM lamella for simultaneous irradiation. Periodic assessment of crystallinity in each of the three regions via nanobeam electron diffraction reveal, at both room temperature and 600ºC, that free surface (pore) defect sinks are more effective than grain boundaries at capturing radiation-induced defects and delaying the onset of amorphization.
Impact of residual ordering in amorphized pyrochlores on their recrystallization behavior
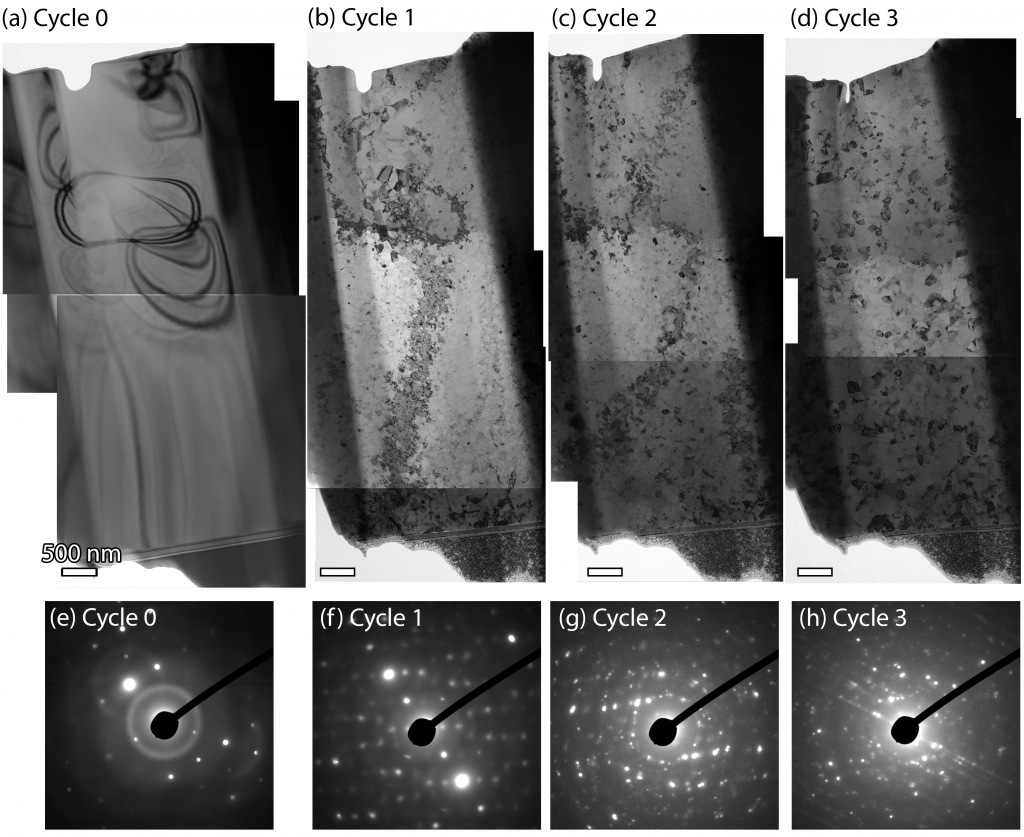
Through a combination of in-situ ion irradiation and in-situ heating within the TEM we have documented the crystallization behavior of gadolinium titanate through repeated irradiation campaigns. The crystallization behavior of the pyrochlore structure was also studied at different levels of helium implantation. The kinetics of the crystallization was observed, revealing different morphologies and therefore rates of crystallization, depending on the total absorbed energy. This work shows that short range order can still exist in the pyrochlore crystal structure despite apparent amorphization. However, there is still much to do to understand the nature and source of this apparent short ranged order.
Acknowledgements
Many have contributed to this research effort, which was lead by former graduate student Nathan J. Madden and now carried on by Nachiket Shah.
The work was supported by the grant DE‐SC0015894 funded by the U.S. Department of Energy, Office of Science and by a U.S. Department of Energy (DOE) Office of Science Graduate Student Research (SCGSR) award.
Samuel A. Briggs, Diana Perales, Timothy J. Boyle, Khalid Hattar have all contributed to this effort while at Sandia National Laboratories given that this work was performed, in part, at the Center for Integrated Nanotechnologies, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science. Sandia National Laboratories is a multi-mission laboratory managed and operated by National Technology & Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International, Inc., for the U.S. DOE’s National Nuclear Security Administration under contract DE-NA-0003525. The views expressed in the article do not necessarily represent the views of the U.S. DOE or the United States Government.
M. Li, W-Y. Chen, and P. Baldo also provided invaluable technical insight and help with operation of the Intermediate Voltage Electron Microscopy – Tandem Facility (IVEM) at Argonne National Laboratory, which was made possible through the by the U.S. Department of Energy, Office of Nuclear Energy under DOE Idaho Operations Office Contract DE-AC07- 051D14517 as part of a Nuclear Science User Facilities experiment.